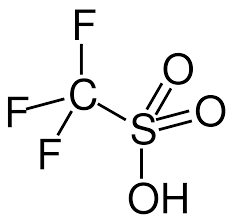
What is Triflic Anhydride?
Triflic anhydride, scientifically known as trifluoromethanesulfonic anhydride, is a powerful reagent commonly used in organic chemistry. With its molecular formula (CF3SO2)2O(CF_3SO_2)_2O, this compound stands out due to its exceptional reactivity and versatility.
Chemical Properties and Formula
Triflic anhydride is a colorless to pale yellow liquid with a sharp, pungent odor. It is highly reactive, particularly as a dehydrating and activating agent in various chemical reactions.
Importance of Triflic Anhydride in Chemistry
Its Role in Organic Synthesis
In organic chemistry, triflic anhydride is indispensable for facilitating reactions like esterification and sulfonation. It acts as a powerful electrophile, enabling the creation of triflates, which are crucial intermediates in complex synthesis pathways.
Key Applications in Chemical Processes
Triflic anhydride’s ability to introduce triflate groups has made it a preferred choice in pharmaceuticals, agrochemicals, and advanced material synthesis.
Applications and Benefits of Triflic Anhydride
Triflic Anhydride in Pharmaceuticals
Catalysis in Drug Development
In the pharmaceutical sector, triflic anhydride is used as a catalyst to improve the efficiency of reactions. Its role in enhancing the production of key intermediates contributes to cost-effective drug manufacturing.
Enhancing Reaction Efficiency
This reagent significantly reduces reaction times, making industrial-scale production faster and more reliable.
Use in Agrochemicals
Role in Producing Herbicides and Pesticides
Triflic anhydride aids in synthesizing active ingredients for herbicides and pesticides. Its high reactivity ensures the production of pure and potent chemical compounds.
Triflic Anhydride in Materials Science
Application in Polymer Development
Materials science leverages triflic anhydride for creating advanced polymers with unique properties, such as high thermal and chemical resistance.
Use in Specialty Coatings
The compound is also instrumental in developing specialty coatings that offer superior protection against corrosion and wear.
How Triflic Anhydride is Produced
Industrial Synthesis Methods
Key Raw Materials
The primary raw materials for triflic anhydride production include triflic acid and anhydrides.
Production Process Overview
Industrial production involves a reaction between triflic acid and a dehydrating agent, yielding triflic anhydride as the primary product.
Safety Precautions in Handling and Storage
Hazardous Nature of Triflic Anhydride
Triflic anhydride is highly reactive and corrosive, posing risks during handling. Direct contact or inhalation can lead to severe health issues.
Recommended Safety Guidelines
Proper storage in airtight containers, use of protective gear, and adherence to safety protocols are essential to minimize risks.
Environmental Impact and Sustainability
Challenges in Disposal
Potential Environmental Hazards
Improper disposal of triflic anhydride can lead to soil and water contamination, posing ecological risks.
Methods for Safe Disposal
Neutralization and incineration are common disposal methods to mitigate environmental harm.
Advances in Green Chemistry
Reducing Environmental Footprints
Research into green chemistry has led to alternative processes that reduce the environmental impact of triflic anhydride production.
Sustainable Alternatives to Triflic Anhydride
Efforts are ongoing to develop reagents with similar reactivity but fewer ecological drawbacks.
Conclusion
Summary of Triflic Anhydride’s Role in Industry
Triflic anhydride is a cornerstone reagent in various industries, from pharmaceuticals to materials science, due to its unmatched versatility and efficiency. Triflic derivatives, such as triflic acid and triflates, are crucial reagents in organic synthesis due to their high reactivity.
Future Perspectives
As industries strive for sustainability, the development of greener alternatives and improved handling practices will shape the future of triflic anhydride usage.
FAQs
What are the primary uses of triflic anhydride?
It is widely used in pharmaceuticals, agrochemicals, and material sciences for its role in enhancing reaction efficiency.
How is triflic anhydride stored safely?
Store it in airtight containers in a cool, dry place, and always use protective equipment when handling.
Can triflic anhydride be replaced with greener alternatives?
Research is ongoing to develop sustainable alternatives that mimic its reactivity with fewer environmental concerns.
What industries rely heavily on triflic anhydride?
The pharmaceutical, agrochemical, and materials science sectors are the primary users of this compound.
Are there environmental concerns linked to triflic anhydride production?
Yes, improper disposal and production can lead to environmental hazards, but advancements in green chemistry are addressing these concerns.