Introduction
Urticaria, commonly known as hives, is a skin condition characterized by itchy, red welts that can vary in size and appear anywhere on the body. While acute urticaria is often short-lived, chronic urticaria persists for six weeks or longer and can significantly impact a patient’s quality of life. The global urticaria market has been experiencing substantial growth, driven by increased awareness, advancements in treatment options, and a rising prevalence of the condition.
Source – https://www.databridgemarketresearch.com/reports/global-urticaria-market
Market Overview
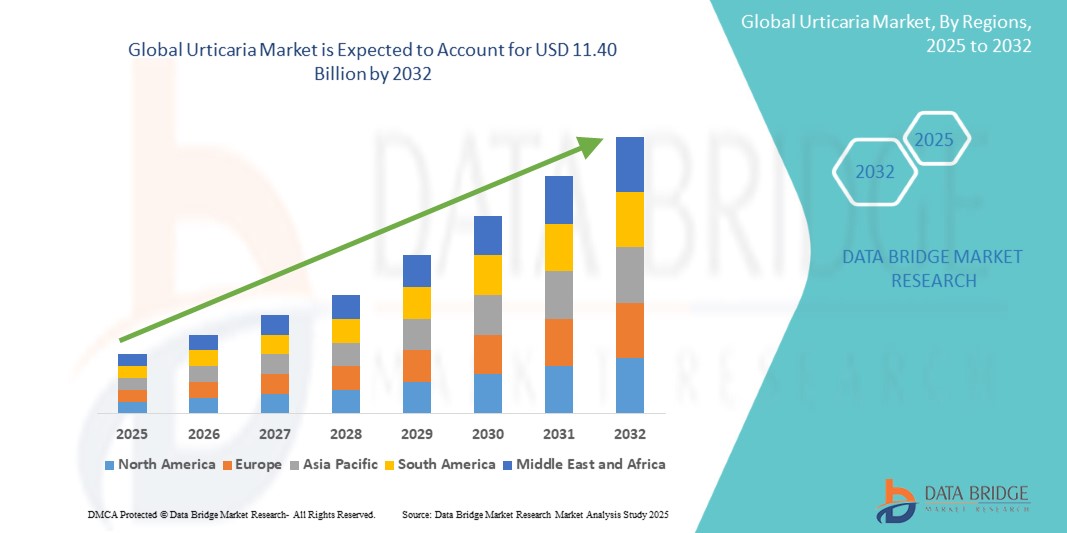
The global urticaria market was valued at approximately USD 3.73 billion in 2024 and is projected to reach USD 11.40 billion by 2032, growing at a compound annual growth rate (CAGR) of 15.00% during the forecast period of 2025 to 2032.
In the top seven major markets (United States, EU4, United Kingdom, and Japan), the chronic urticaria market reached a value of USD 15.1 million in 2024 and is expected to grow to USD 36.3 million by 2035, exhibiting a CAGR of 8.31% during 2025-2035.
Key Market Drivers
1. Rising Prevalence of Urticaria
The increasing incidence of urticaria, particularly chronic spontaneous urticaria (CSU), is a significant driver of market growth. Factors such as environmental changes, stress, and autoimmune disorders contribute to the rising prevalence.
2. Advancements in Treatment Options
The development of innovative therapies, including biologics like omalizumab and dupilumab, has revolutionized urticaria treatment. These targeted therapies offer improved efficacy and safety profiles compared to traditional antihistamines.
3. Growing Awareness and Diagnosis Rates
Increased awareness among healthcare providers and patients has led to earlier diagnosis and treatment initiation, contributing to market expansion. Educational campaigns and improved diagnostic tools have played a crucial role in this trend.
4. Strategic Collaborations and Approvals
Pharmaceutical companies are engaging in strategic collaborations and acquisitions to enhance their product portfolios. For instance, in March 2025, Novartis acquired the rights to Kyorin’s CSU candidate, KRP-M223, in a deal valued at $830 million. Additionally, in April 2025, the U.S. FDA approved Dupixent (dupilumab) for treating CSU in adults and adolescents aged 12 years and older who remained symptomatic despite antihistamine treatment.
Market Segmentation
By Treatment Type
-
Antihistamines: First-line therapy for urticaria, providing symptom relief.
-
Leukotriene Receptor Antagonists: Used in combination with antihistamines for enhanced efficacy .
-
Monoclonal Antibodies: Targeted therapies like omalizumab and dupilumab for refractory cases.
-
Immunosuppressants: Employed in severe cases unresponsive to other treatments.
By Route of Administration
-
Oral: Common for antihistamines and leukotriene receptor antagonists.
-
Parenteral: Used for biologics and immunosuppressants.
By End-User
-
Hospitals: Primary centers for diagnosis and treatment initiation.
-
Specialty Clinics: Focused care for chronic and refractory cases.
-
Homecare: Growing trend with the availability of self-administered therapies.
Regional Insights
North America
North America dominates the urticaria market, attributed to advanced healthcare infrastructure, high awareness levels, and the presence of key market players. The region’s market is bolstered by favorable reimbursement policies and ongoing clinical research.
Europe
Europe holds a significant market share, driven by increasing prevalence and adoption of advanced therapies. Collaborative efforts among countries for research and development further support market growth.
Asia-Pacific
The Asia-Pacific region is expected to witness the fastest growth due to rising awareness, improving healthcare infrastructure, and increasing healthcare expenditure. Countries like China and India are emerging as key markets.
Latin America and Middle East & Africa
These regions are experiencing gradual growth, with increasing investments in healthcare and rising awareness about urticaria and its treatments.
Competitive Landscape
The urticaria market is highly competitive, with several key players focusing on research and development to introduce novel therapies. Major companies include:
-
Novartis Pharmaceuticals
-
Sanofi
-
Regeneron Pharmaceuticals
-
Roche
-
AstraZeneca
-
Amgen
-
Allakos
-
Incyte Corporation
These companies are investing in clinical trials and strategic partnerships to expand their product offerings and market presence.
Challenges and Opportunities
Challenges
-
High Treatment Costs: Biologic therapies are expensive, limiting accessibility in low-income regions.
-
Limited Awareness in Developing Regions: Lack of awareness hampers early diagnosis and treatment initiation.
-
Regulatory Hurdles: Stringent regulations can delay the approval and launch of new therapies.
Opportunities
-
Emerging Markets: Untapped markets in Asia-Pacific and Latin America offer significant growth potential.
-
Technological Advancements: Innovations in diagnostics and treatment delivery can enhance patient outcomes.
-
Personalized Medicine: Tailoring treatments based on individual patient profiles can improve efficacy and reduce adverse effects.
Future Outlook
The urticaria market is poised for substantial growth, driven by ongoing research, increasing prevalence, and advancements in treatment options. The shift towards personalized medicine and the development of novel biologics are expected to transform the treatment landscape. With strategic collaborations and investments, the market is likely to witness the introduction of more effective and accessible therapies, improving the quality of life for patients worldwide.